|
|
|
Booklet of abstractsSession 1 :Gabriel Amselem This talk will cover 2 different projects studied in the lab, involving the phototaxis of Chlamydomonas reinhardtii. In the first project, we study the phototaxis of Chlamy. We show experimentally that Chlamy has a short-time memory of its past stimuli, which leads it to overreact to light, as if it were integrating consecutive light stimuli over time. This is the exact opposite behaviour of the well-known light adaptation, where past stimuli rather desensitise the algae. A generic biochemical model is proposed to qualitatively explain this behaviour. In the second project, we place Chlamy and plastic beads of diameters up to 500 microns in a centimetric well. We show that by inducing the accumulation of algae in well-defined regions using phototaxis, we can generate macroscopic flows, and so accumulate and transport beads in a controlled manner to defined locations.
A. Givaudana, H. de Maleprade, F. Picella The unicellular alga Chlamydomonas has two flagella whose beating enables it to swim in a breast stroke. Chlamydomonas is bottom heavy, which reorients its spontaneous swimming to the top. Moreover, like plants, algae get energy from photosynthesis and to assure this behavior, they swim towards light. This natural bioconvection favours the accumulation of a layer of denser cells at the free surface, with subsequently destabilises into sinking plumes [1]. By studying the effect of light on plume patterns, we observed in addition to the plume migration, the emergence of a periodic structure along the illuminated wall. This behavior highlights the formation of a new hydrodynamic instability in a bio-active fluid. But, what drives this symmetry breaking ? Where do these structures come from ? We assume that they follow the rise of a roll at the wall driven by phototactic swimming of the algae. The experimental set-up is shown on the schematic (Fig. 1 (a)). We vary the light intensity, solution depth and Chlamydomonas concentration. Fig. 1 (b) and (c) show that the wavelength Fig.1 – (a) Experimental set up is placed under a big dark box to ensure good control of light rays. A smaller box containing Chlamydomonas solution is illuminated by a blue lamp and a red lamp illuminates from below, as these micro-algae are not sensitive to red light. A camera records the top view. (b) Top view for a 3mm solution depth. (c) Top view for a 6mm solution depth. λ is the wavelength inside the roll and L is the roll width. [1] Martin A. Bees, Advances in Bioconvection, Annual Review of Fluid Mechanics, 449-476, 2020
Paula Araujo Gomes1,2,3, Jean-Baptiste d’Espinose de Lacaillerie1,2, Bruno Lartiges4 , Martin Maliet1 , Valérie Molinier3,5, Nicolas Passade-Boupat3,5, and Nicolas Sanson1,2 One of the major bottlenecks for microalgae production is the harvesting process because of the colloidal stability of unicellular algae in suspension [1, 2]. Interaction forces between particles in an electrolyte solution govern the colloidal stability of a suspension. Therefore, the comprehension of these interactions is fundamental to optimizing the harvesting of microalgae suspensions. It is well known that the electric double layer plays a fundamental role in the electrostatic stabilization of colloids. Soft particle theory developed mainly by Ohshima [3, 4] describes the electric properties of particles covered by an ion-permeable polyelectrolyte layer, which is usually the case for biological particles [5]. In this study, the influence of extracellular permeable layer on the electrokinetic properties was examined for one freshwater microalga, Chlorella vulgaris, and two seawater microalgae species, Nannochloropsis oculata and Tetraselmis suecica. The soft particle model was used to interpret the electrophoretic mobility measurements at different ionic strengths. This allowed us to estimate two parameters that define the characteristics of the polyelectrolyte layer of each microalga: volume charge density and the characteristic penetration length. These parameters were determined from the fitting of the measured electrophoretic mobilities to Ohshima’s theory and then evaluated by a sensitivity analysis. Furthermore, transmission electron microscopy (TEM) was performed to observe the cells’ surface and analyze the polyelectrolyte layer of microalgae. The results showed that all three microalgae have a soft particle character. Additionally, the surface potential of microalgae was estimated from electrophoretic mobility measurements using the soft particle theory, showing that the algae surface potential is less negative than the apparent zeta potential [6]. This finding indicates that the energy barrier due to the electrostatic repulsion between cells is much smaller than that usually expected [7]. References [1] Garrido-Cardenas et al. (2018). Algal Research, 35, 50-60. [2] Vandamme, Foubert, & Muylaert (2013). Trends in Biotechnology, 31(4), 233-239. [3] Ohshima (1995). Advances in Colloid and Interface Science, 62(2-3), 189-235. [4] Maurya et al. (2020). Journal of Colloid and Interface Science, 558, 280-290. [5] Makino & Ohshima (2011). Science and Technology of Advanced Materials, 12(2), 023001. [6] Gomes et al. (2022). Langmuir, 38(46), 14044-14052 [7] Hori & Matsumoto (2010). Biochemical Engineering Journal, 48(3), 424-434. Bruno Ventejou1, Thibaut Métivet2, Aurélie Dupont1 and Philippe Peyla1 We use a simple model of swimmer inspired from literature of micro-swimmers moving in a viscous liquid (the so-called Stokes regime). However, we numerically explore the model, even at intermediate and high Reynolds numbers where inertia prevails over viscous force. As a result, we obtain universal laws that can be recovered by scaling arguments. From the Stokes to turbulent regimes, our results compare very well with experimental data previously published on millimeter to meter size aquatic species. We have also collected data on a wide variety of micro-organisms that corroborate our numerical results.
Salima Rafaï A study of the light-induced evacuation of a suspension of microwimmers (the micro-alga Chlamydomonas reinhardtii) through a bottleneck-shaped constriction is presented. We show that a transition from a jammed phase to an uninterrupted phase occurs when varying either the door size or the swimming velocity. The survival function of exit times is then found to evolve from a powerlaw to an exponential trend. Moreover, we found that the evacuation time increases with increasing velocity, a behavior reminiscent of the "faster-is-slower" paradox present in crowd dynamics systems.
Session 2 :Aliénor Lahlou and Benjamin Bailleul
Marcelo ORLANDO1, Sandrine BUJALDON1, Andrea LODETTI2, Vincent CROQUETTE3, Ludovic JULLIEN4, Shizue MATSUBARA2, Ladislav NEDBAL5, Julien SELLÉS1, Benjamin BAILLEUL1 Photosynthetic organisms continuously face the challenge of adapting to ever-changing environments [1]. Whether it’s fluctuations in light intensity or in CO2 availability, the photochemical phase of photosynthesis adjusts to ensure that the metabolic requirements for NADPH and ATP are constantly fulfilled and that photosystems are protected from the harmful consequences of light excess. This complex balance involves precise modulation of electron, proton and ion fluxes, orchestrated by various known regulatory mechanisms [2]. However, the question of precisely how these mechanisms coordinate and interact with each other remains unanswered. Recent studies have highlighted the effectiveness of “undulatory photosynthesis” — using oscillating light and frequency domain analysis [3] — as a powerful tool for understanding the orchestration of photosynthetic regulation [4]. By leveraging established techniques in the fields of control and systems engineering, these methods seek to bridge the gap between meticulously controlled laboratory conditions and the multifaceted realities of natural environments. Inspired by this methodology, we subjected the green alga Chlamydomonas reinhardtii to sinusoidally modulated light, spanning frequencies from 10-2 to 102 Hz. We assessed two key observables of the photosynthetic apparatus: the electro-chromic shift, indicative of proton and ion fluxes across the thylakoid, and chlorophyll a fluorescence, informing on electron flows. Our analysis, depicted through Bode diagrams based on these observables, reveals that photosynthesis behaves akin to a low-pass filter, in the sense that it is unable to track faster light oscillations. However, intriguingly, more intricate patterns emerge at lower frequencies, potentially associated with specific regulatory mechanisms operating within the corresponding timescales. To delve deeper into these phenomena, we employ specific photosynthetic mutants, particularly those influencing alternative electron flows and ion channels. As part of the EIC-Pathfinder DREAM project, “undulatory photosynthesis” serves a dual purpose: improving our understanding of the coordination between the fundamental modules of photosynthetic regulation; and developing a stress diagnostic tool based on "frequency signatures" associated with different stress factors such as nutrient deficiencies, high light or low temperatures. [1] Matsubara, S. Journal of Experimental Botany (2018). [2] Croteau, D., Alric, J. & Bailleul, B. The Chlamydomonas Sourcebook (2023). [3] Nedbal, L. & Březina V. Biophysical Journal (2002). [4] Niu, Y., et al. New Phytologist (2023). G. Jacucci1, J. F. Allemand1, S. Gigan2, R. Jeanneret1 Chlamydomonas reinhardtii cells have the ability to orient themselves in light fields, a property called phototaxis. As opposed to chemotaxis (the capacity to navigate chemical fields) which has been extensively studied in the past 50 years, this phenomenon, mediated by a specialised organelle called the eyespot, is still poorly understood. In this work, we studied the swimming behaviour of micro-algae in tailored laser illuminations in confined microfluidic chambers. By tracking individual cells, we demonstrated that the run-and-tumble motion of Chlamydomonas is influenced by both local light intensity and gradient. Notably, under ring-shaped illumination, the micro-algae climb the light gradient and accumulate inside the ring, primarily due to an increase in tumbling frequency with light intensity. These findings offer new insights into how Chlamydomonas modulates its motility in response to light, adapting to both its direction and gradient. Our experiments also propose novel strategies for controlling and redirecting their movement.
Guillaume Allorent Photosynthetic organisms encounter daily fluctuations in light within their natural habitats. While light serves as a vital energy source for photosynthesis, it can also pose a risk of photodamage. Thus, the delicate balance between light absorption and dissipation is essential for their survival and overall fitness. These organisms have developed various strategies to acclimate and respond to these light fluctuations. Among these responses are complementary mechanisms that either prevent, limit, or repair photodamage. These mechanisms are triggered at different intervals upon detecting changes in light conditions. One highly effective photoprotective mechanism is the process of dissipating light energy through non-photochemical quenching (NPQ), which activates within seconds. NPQ plays a critical role in enabling survival under excessive light conditions. Its activation relies on specific Light-Harvesting Complex (LHC) proteins. In the case of Chlamydomonas reinhardtii, the expression of these proteins is influenced not only by light intensity but also by the color of light. This sensitivity to light color stems from the activation of photoreceptors that monitor the spectrum of light in the environment, ranging from UV-B to far-red light.In this presentation, I will discuss how Chlamydomonas finely tunes the balance of light intensity and color to optimize its response to light.
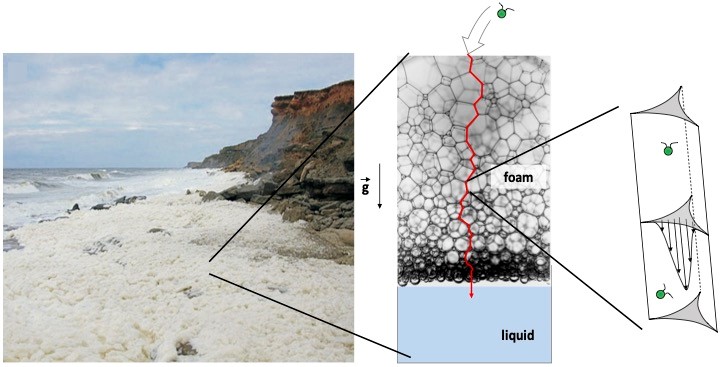
Session 3 :Florence Elias A massive formation of stable sea foam is regularly observed on certain coastlines. These naturally occurring foams are associated with a loss of phytoplankton biomass and biodiversity in the seawater. We are investigating whether the phytoplankton advected into the foam during its formation remains trapped in the complex network of internal channels in the foam. In this talk, I will present experiments carried out in the laboratory to study the retention in a liquid foam of a model phytoplankton organism: the unicellular alga Chlamydomonas reinhardtii (CR), which is bi-flagellate and therefore motile. We measured the escape dynamics of CR cells from the foam. A comparison between live and dead cells shows that live CR cells tend to be retained in the foam. Finally, I will discuss the microscopic mechanisms that can lead to this entrapment, which raises the question of the transport of microswimmers in confined and environments under flow.
A. N. Kato1, K. Xie1,2, B. Gorin1, J-M. Rampnoux1, and H. Kellay1 Keywords: active matter, interface, droplet, thermal Marangoni effect.
Elettra Figa Talamanca, Hélène de Maleprade, Juliette Pierré Marine aerosols are crucial to ocean/atmosphere exchanges; they are produced during the explosion of the many surface bubbles [1]. Some of these micro-droplets travel great distances: their content spreads over a wide area. The ocean surface is covered with surfactants, biological matter and particles. We have recently demonstrated that the presence of surfactants radically modifies the jet emitted during the explosion of a surface bubble: aerosols are either inhibited or enhanced in comparison to the well-studied case of the explosion of a bubble in a pure liquid [2]. It is known that microorganisms modify the effective viscosity of a liquid locally: how do they impact aerosol production? Are they captured and subsequently propagated over long distances? To answer to those questions we built an experimental setup that allow us to study the aerosol formation in a solution of Chlamydomonas reinhardtii: we inject an air bubble in the solution with a syringe pump; a high-speed camera follows the bubble bursting phenomenon, and we measure physical parameters as bubble size, drop size and drop velocity. We catch the first and speeder drop with a glass slide above the solution. We check the presence the presence of micro-organisms with the microscope. In this presentation we will discuss the size and the velocity of the first droplet, as well as the presence and mobility of microalgae in the droplet. Figure 1: Left: Experimental setup. Right: Chlamydomonas in the first droplet. References [1] L. Deike, Mass Transfer at the Ocean–Atmosphere Interface: The Role of Wave Breaking, Droplets, and Bubbles Annu. Rev. Fluid Mech. 54: 191–224 (2022) [2] J. Pierre, M. Poujol and T. Seon, Influence of surfactant concentration on drop production by bubble bursting PRF 073602-1 (2022)
Jean-Baptiste d'Espinose de Lacaillerie Microalgal cell flocculation is a crucial aspect of microalgae cultivation and harvesting.1 Despite the thorough examination of cell surface properties,2 the specific processes responsible for microalgal flocculation under high pH conditions, commonly linked to sweeping flocculation,3 have not been completely elucidated at the cellular scale. Our study focuses on investigating the dynamics of microalgal cell aggregation, e.g. for Chlorella vulgaris in seawater at elevated pH levels. Our investigations linked observations at two different scales. At the macroscopic scale, the settling kinetics of algal suspensions was related to algae aggregate microstructures. At the cellular scale, microalgal cell adhesions was measured in seawater growth media using micropipette force sensors.4 At both scales, findings indicated that cell aggregation occurred at high pH through heteroaggregation by hydrolysis and precipitation of the alkali-metal ions (Mg2+ and Ca2+) naturally present in seawater. It was demonstrated that the degree hydrolysis, by determining the proportion of precipitate to cells, significantly impacts the type of mechanism: cell clustering promoted by hydroxide surface precipitation or cell entrapment within a hydroxide gel. This underscores the pivotal role of water inorganic chemistry in the aggregation process.
|